Ivermectin in Itajaí: researchers reaffirm efficacy and provide raw data for the scientific community
"One of the most remarkable studies in the history of medicine" now has its public data for worldwide scrutiny.

The largest Ivermectin study in the world, conducted in Itajaí, Santa Catarina, in southern Brazil, recently classified by Dr Pierre Kory, professor of medicine and one of the world's leading experts in intensive care, as "one of the most remarkable studies in the history of medicine" in a recent US Senate hearing, now has its data publicly available to the world scientific community.
The researchers' goal in making available the raw data, produced by the public structure of the SUS - Sistema Único de Saúde ( National Health System), is transparency. "Those who do not owe do not fear. It's not because lack of transparency has been the rule in vaccine and other drug research at COVID that we would do the same," said Dr Flavio Cadegiani, one of the scientists who co-authored the study
"I challenge anyone who dares to question the data to go to Itajaí and get the raw data from the SUS system itself," he added.
The research, published on January 15 in the prestigious scientific journal Cureus after international peer review, involved the entire population of Itajaí in an action designed to reduce the impact of the COVID-19 pandemic throughout the city. (Read details of the study in Portuguese and English).
The study found efficacy at every stage: among citizens who chose to take preventive ivermectin there was a 44% reduction in the chance of being infected with the virus. Among those who took the drug and still got infected, there was a 56% reduction in the chance of being hospitalized, compared with those who did not. And among those who took it preventively, the chance of death from COVID-19 compared to those who did not take it was reduced by 68%.
Publishing the data aims to fulfill a BMJ request for transparency
Making the data publicly available comes at the same time that an editorial in the BMJ (British Medical Journal), one of the world's most respected medical journals, published on January 19, called for all raw data from vaccines and new treatments for COVID to be made public. "In my view, the BMJ has made it very clear. Not taking the data public, even on demand, is an admission of fraud," said Dr Cadegiani.
"We would like all research in COVID, not just on repositioned drugs, to have the level of transparency that we have. This is the desire of those who seek scientific truth," he added.
Scientists praise transparency
"It is excellent that they released their anonymized data. They have true confidence in the results of their analyses. That is how good science is supposed to be carried out.", said Dr Harvey Risch, professor of epidemiology at Yale University, USA, emphasizing that the patients' personal data are preserved.
Data scientist Lorenzo Ridolfi, PhD in computer science, who has been analyzing data and waves of the pandemic's impact in Brazil for the website zerobias.info, praised the action. "It is very difficult to find data on vaccines and other treatments for COVID. If all studies had this openness, we would have much more transparency in the results."
Transparency that vaccines don't have
The publication of the data occurs at the same moment in which the scientific community speculates about the resignation of Ricardo Palacios, head of research of Coronavac, a vaccine with 84.2 million doses applied in the Brazilian population, which even after a year, still has no published study or public data. Palacios has stepped down from the Butantan Institute, the Brazilian public entity that is the manufacturer's partner in the effort to vaccinate against COVID-19.
"The Coronavac study is just one example of the asymmetry of treatment by the media. What is behind this inequality?", questions Dr Cadegiani.
The data is available to anyone, but it preserves the identity of the citizens of Itajaí. "The data is there for anyone who wants to recalculate. Even the most arduous statistical gymnastics will not be able to undo the effectiveness of the drug," he said.
"We want to be a world case of transparency and thus encourage universities and scientists around the world to go in this direction," added Dr Cadegiani.
Scientific community bet against making the data available
Publishing the data is so unprecedented in science that Kyle Sheldrick, an Australian physician and researcher who is one of the biggest critics of the ivermectin studies, recently bet: "they will renege on multiple previous commitments to publicly post the dataset and claim it's now on request".
"Once again they bet wrong. The purpose behind all this is clear. But when the study speaks for itself, no one can stop it. Interesting that these people have never asked for data on patented products," said Cadegiani.
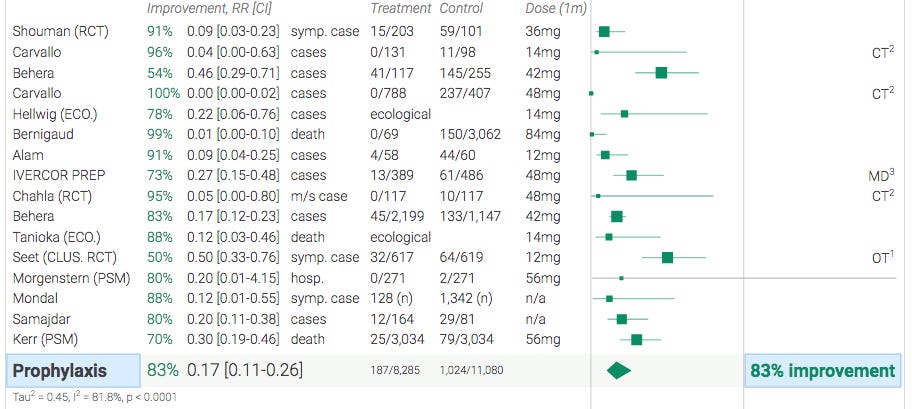
Itajaí only confirmed previous studies
There are 15 other studies on prophylaxis with ivermectin, a cheap, generic, off-patent drug. All of them are unanimously positive. However, since Itajai was with a large number of patients and uses the statistical technique PSM - Propensity Score Matching, due to the data having great precision, there is a greater weight in this study as scientific evidence. In other words, Itajai only confirmed other studies.



Bravo!
The latest Dr. Cadegiani's and Dr. Kerrs' paper compared the <60mg irregular users and >180mg regular users vs. non-users of ivermectin.
Here is a small contribution about the intermediate 60-180mg group. It makes the conclusion rock solid.
https://massimaux.substack.com/p/the-reduction-in-infection-and-covid